Which Continuous Improvement Method Does Cpk Measure
February 2012
Does your organization have a process for improving process capability? Or does your organization calculate Cpk values each month? Do you know why this monthly calculation is wrong to do? Or does your organization simply look at % out of specification? Or does your organization rely simply on Black Belts to figure it out?
This month's newsletter examines a process for improving process capability. It starts with selecting a Key Output Variable (KOV) - a specification that is important to a customer. It can be, for example, weight, size, purity, density, or on-time delivery - something that is critical to the customer. The methodology examines sampling and the test method. It also includes, of course, a control chart to monitor the KOV over time.
The process capability is calculated and compared to the goal for Cpk. If the goal is not being met, Key Input Variables (KIVs) are identified. KIVs are those knobs in the process that you turn to impact the KOV. Experimental design techniques (DOE) are then used to determine which KIVs have a significant impact on the KOV (both the average and the variability). Process changes are made based on the DOE results. The methodology continues until the goal for Cpk is reached - or you determined you just plain can't do it with the current process.
In this issue:
- Process Capability Review
- The Methodology
- Summary
- Quick Links
You may add your comments after the newsletter.
Process Capability Review
Process capability is a measure of how well your process meets specifications. Cpk is the value most used to represent process capability. Cpk is the minimum of the capability based on the upper specification, Cpu, and the capability based on the lower specification, Cpl. Cpu and Cpl represent how far the upper and lower specification limits are from the average in terms of 3 sigma. The figure below shows the Cpk calculations.
Figure 1: Cpk Review
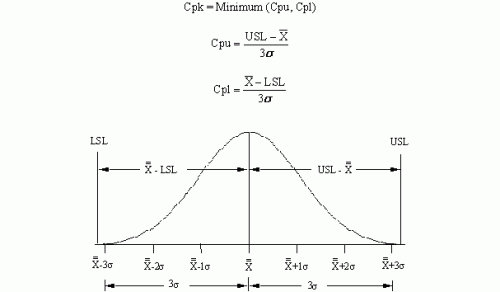
If you need a more in-depth review of process capability, please take a look at our three-part series on process capability from late 2004.
The Methodology
The steps in the methodology are shown in Figure 2 (download picture as PowerPoint file). The steps are described below. The methodology works for any type of process, although certain adaptations will be needed depending on your process.
Figure 2: Cpk Improvement Methodology
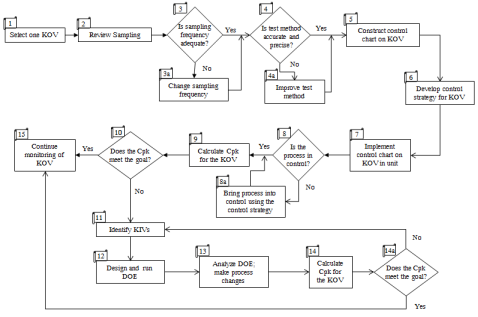
Step 1: Select One KOV
 Key Output Variables (KOVs) are those product characteristics that have a significant impact on customer satisfaction. Too much variation in the KOVs can lead to problems with quality and costs. Examples of KOVs include the amount of water in a bottle, chemical purity, part dimensions, or color of a resin.
Key Output Variables (KOVs) are those product characteristics that have a significant impact on customer satisfaction. Too much variation in the KOVs can lead to problems with quality and costs. Examples of KOVs include the amount of water in a bottle, chemical purity, part dimensions, or color of a resin.
To start the methodology, select one KOV. You may have already calculated Cpk values for many your KOVs. If you have, start with a KOV whose Cpk is below what the customer wants. You should also set the goal at this time. In most cases, it will simply be the Cpk that the customer is requesting. Today, most customers want Cpk values of 1.33 or over. The requirement can be much greater for safety or medical products.
Step 2: Review Sampling
Examine how and where the process is sampled. For a KOV, the sample should be taken at the end of the process. This is easy to determine most of the time, for example, if your process has a single machine or is a small cell of machines. In some industries, like the chemical industry, the value of the KOV changes throughout the process. You want to sample the KOV at the upstream point where it will not change anymore. This may often be when it is ready for shipping - for example, in railcars or trucks.
Step 3: Is Sampling Frequency Adequate?
Examine how often the process is sampled. Is it sufficient? Quite often, the Cpk value can be used to help determine how often you sample. Assuming you are working on a KOV whose Cpk needs to be improved, you probably should be sampling more often than less. If your Cpk is less than 1, you are not capable of producing everything within specifications. In that case, you probably should be doing 100% inspection to ensure that out of specification product is not getting through. If the sampling frequency needs to be change, change it (step 3 A).
Step 4: Determine if Test Method is Precise and Accurate
 After sampling, the next step is to look at the test method. Is it capable of doing what you want? Can it distinguish between samples? This step involves determining if the test method is accurate and precise. We have several newsletters on test methods including how to monitor test methods using SPC, variable measurement systems (bias, linearity, Gage R&R) and attribute gage R&R.
After sampling, the next step is to look at the test method. Is it capable of doing what you want? Can it distinguish between samples? This step involves determining if the test method is accurate and precise. We have several newsletters on test methods including how to monitor test methods using SPC, variable measurement systems (bias, linearity, Gage R&R) and attribute gage R&R.
In the May 2006 newsletter, we described how to determine the accuracy and precision of the test using an individuals control chart (X-mR). We defined accuracy and precision as:
- An accurate test method is defined as a test method that is in statistical control with respect to the average of the standard (i.e., the X chart) and the center line on the X chart is equal to the true value of the standard.
- A precise test method is defined as a test method that is in statistical control with respect to variation (i.e., the range chart) and that is responsible for less than 10% of the total process variance.
If the test method is not accurate or precise, it must be improved (step 4a) before you can move to improving the process capability. Often, improving the test method improves the Cpk for the KOV.
Step 5: Construct a Control Chart on the KOV
Develop a control chart on the KOV based on historical data. This initial control chart is not ready to be put into the hands of those closest to the process - the ones who should be using the control chart to monitor the KOV. This is an initial chart that is used to set the control limits for the future. Our March 2011 newsletter explains the purpose of control charts. In this case, the initial control chart is made to set the control limits for the future.
Step 6: Develop a Control Strategy for the KOV Control Chart
 Once the control chart is put in the hands of those closest to the process, they need to know what to look for if and when the process goes out of control. This is the purpose of a control strategy.
Once the control chart is put in the hands of those closest to the process, they need to know what to look for if and when the process goes out of control. This is the purpose of a control strategy.
The point an operator plotted on a control chart is above the upper control limit. His chart is telling him that there is a special cause present in the process. He is supposed to find out what caused this point to be above the upper control limit. Where does he start looking? There are thousands of things which could have caused this point to be above the upper control limit. A control strategy provides a method of helping him look for causes of out-of-control points.
Control strategies are specific action plans for bringing a process back into control. The strategies usually consist of five to ten steps that help you find reasons for special causes, and most importantly, help you do something about the causes. Our August 2004 newsletter describes a control strategy in more detail.
Step 7: Implement the Use of the Control Chart
The control chart is now ready to be given to those closest to the process. Of course, they need to trained in common and special causes of variation, in how to interpret control charts and how to use the control strategy. The goal for those closest to the process is to bring the process into statistical control, i.e., only common causes of variation present.
Step 8: Is the Process in Control?
The process has to be brought into control by removing special causes of variation. Bringing a process into statistical control puts it back where it is supposed to be. Now real efforts at process improvement can begin. If the process has special causes of variation present, these should be removed using the control strategy or additional cause and effect tools as needed (step 8a).
Step 9: Calculate the Cpk
 Once the process is in statistical control, calculate the process capability value, Cpk. Yes, you should worry a little about how normal or symmetrical the data are. If the data are very skewed, you may need to use a transformation or distribution fitting. You could also simply take a look at the histogram versus the specifications.
Once the process is in statistical control, calculate the process capability value, Cpk. Yes, you should worry a little about how normal or symmetrical the data are. If the data are very skewed, you may need to use a transformation or distribution fitting. You could also simply take a look at the histogram versus the specifications.
Step 10: Does the Cpk Value Meet the Goal?
If the Cpk value meets the goal, then you are essentially finished. Continue monitoring the KOV using the control chart (Step 15). Do not stop monitoring the KOV. You will lose your gains. If the Cpk value does not meet the goal, then you will need to move towards experimental design techniques to either move the average or reduce the variation in the results to improve the Cpk value. Moving the average is always easier.
Step 11: Identify KIVs
 Key input variables are process inputs that have a significant impact on the KOV. The question in this step is:
Key input variables are process inputs that have a significant impact on the KOV. The question in this step is:
What key input variables (KIV) impact the KOV?
For example, if you are making a chemical product, KIVs could include reaction temperature, residence time or reaction pressure. If you are heat treating steel, KIVs could include line speed, hardening temperature or tempering temperature. To develop the list of KIVs, hold a brainstorming session with those closest to the process and others who have knowledge of the process. Once the list is brainstormed, agree on the major KIVs to be tested using experimental design techniques. The KIVs should be those variables thought to impact the average and/or the variation in the KOV. The range (levels) of the KIVs to be used in the experimental design should also be determined at this point.
Step 12: Design and Run an Experimental Design
 Design an experiment based on the KIVs and their levels. The design should include replications so the impact of the KIVs on variability can be determined. Run the experimental design and analyze the results.
Design an experiment based on the KIVs and their levels. The design should include replications so the impact of the KIVs on variability can be determined. Run the experimental design and analyze the results.
You can do a lot with simple two level experimental designs. We have four newsletters you can review on experimental design.
Step 13: Analyze the Results from the Experimental Design
Analyze the results to determine which KIVs have a significant impact on the KOV. Use that knowledge to adjust the KIVs to optimize the process. This is not as simple as it sounds. You may have to do multiple experimental designs to truly find out what impacts the average and variability of the KOV.
Step 14: Recalculate the Cpk and Compare to the Goal
At this point, the KIVs have been set to the levels determined by the experimental design. Continue to collect data using the control chart. The control chart will indicate what impact the experimental design had. Once you have sufficient data, recalculate the process capability. If it now meets the goal, you are essentially done. You simply continue to monitor the KOV using the control chart (Step 15). If not, you must return to Step 11 to find more KIVs and repeat the experimental designs - or you have to admit your process is not capable of meeting the goal for the Cpk without major changes in design and equipment.
Step 15: Continue to Monitor the KOV
Continue to monitor the KOV using the control chart. Revisit your sampling plan. If your Cpk is large enough, you may be able to do less sampling.
Summary
This newsletter began with a brief review of process capability. A 15 step methodology for improving process capability was introduced. The methodology included examining sampling as well as the accuracy and precision of the test method. Experimental design techniques are one key to improving the process capability.
Source: https://www.spcforexcel.com/knowledge/process-capability/cpk-improvement-methodology